Due to a slip-up on the FDA’s front, 600,000 bottles of the commonly used medication are being pulled from shelves. Due to ingredients used by an unregulated manufacturer, the medication is being recalled due to a fear of possible contamination. It’s Ramipril capsules in strengths of 2.5 mg, 5 mg, and 10 mg.
This particular medication is prescribed for hypertension. It is used to open the blood vessels and reduce heart pressure. It is usually administered after a heart attack.
One of the ingredients was being used by a factory in India with improper checks done for their suppliers. The FDA thinks the risk is low, but, better safe than sorry. Currently, there have been no adverse effects. However, if you have one of the listed bottles, best to throw it out.

Related Posts
admin
·
February 28, 2026
·
The hip-hop world is pausing to remember Oliver Power Grant, a pivotal architect behind the early momentum of Wu-Tang Clan. Though rarely in the spotlight, Grant helped guide the…
admin
·
February 28, 2026
·
Living with diabetes means paying attention not only to blood sugar levels but also to how your circulation is functioning throughout the body. According to the American Diabetes…
admin
·
February 28, 2026
·
Noticing that the veins in your hands look more pronounced than usual can feel surprising at first. Before jumping to conclusions, start with perspective. In many cases,…
admin
·
February 28, 2026
·
For decades, drivers have relied on a familiar pattern when shifting gears. Manual transmissions typically feature numbered gears for forward motion along with an “R” for reverse,…
admin
·
February 28, 2026
·
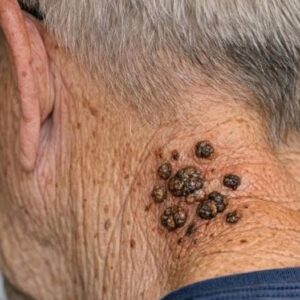
As people age, their skin commonly develops various spots and growths that can look unusual or concerning. While many of these changes are harmless, symptoms like itching…
admin
·
February 28, 2026
·
The United States and Israel carried out coordinated military strikes on Iran early Saturday after diplomatic efforts surrounding Tehran’s nuclear program failed to meet the demands set…